At the turn of the 21st century, scientists Robert Langreth and Michael Waldholz posited a new idea: What if drugs were tailor-made to fit each patient’s genetic profile?
After two decades of scientists and engineers quietly toiling in biotech, quantum theory, artificial intelligence and data science, we are starting to see their theory become a reality.
The way we make and buy drugs today is not unlike how we buy clothing — mass-manufactured and sometimes ill-fitting. The way we are treated by doctors is often inefficient and diagnoses sometimes amount to a physician’s best guess.
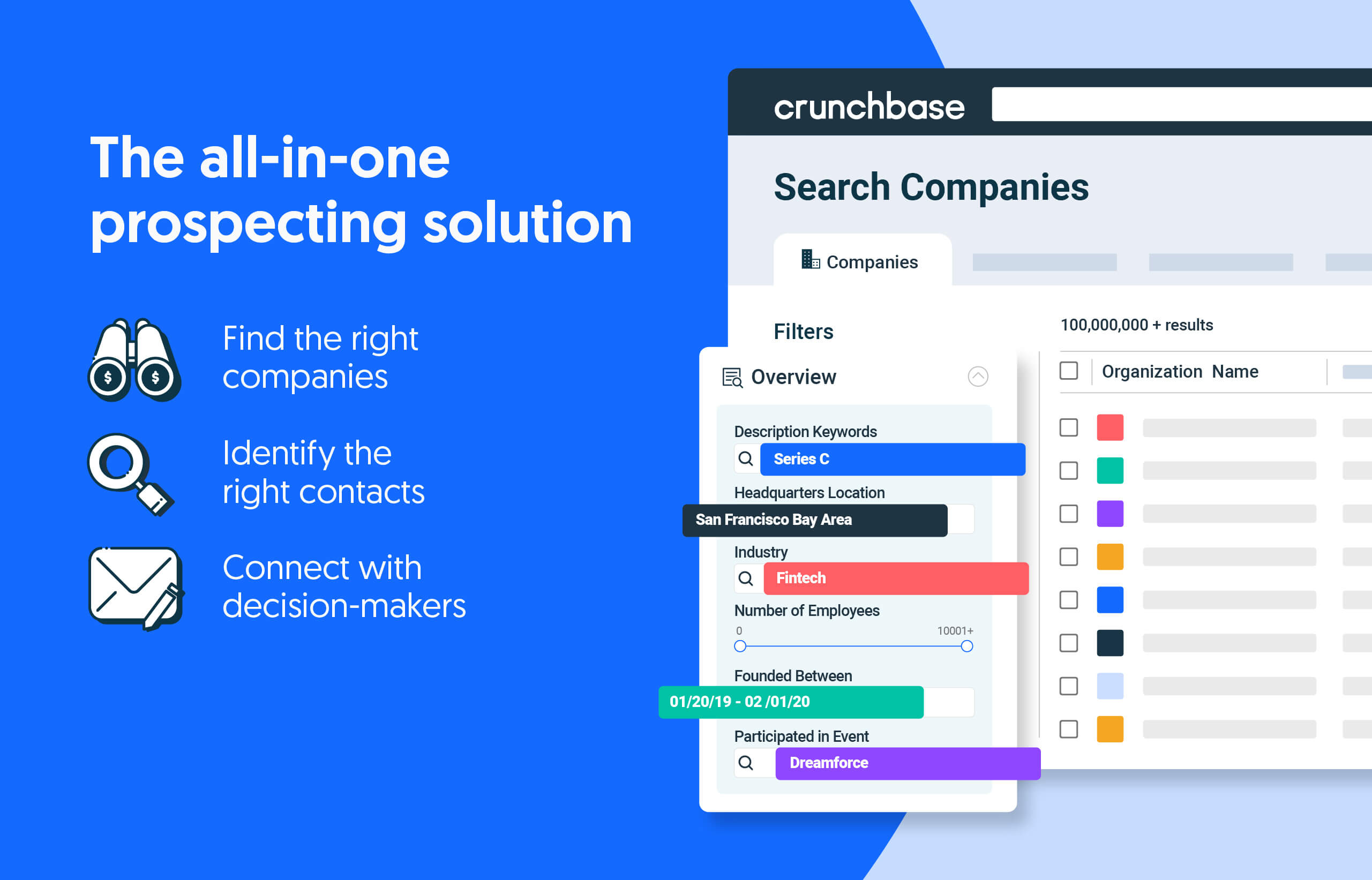
Search less. Close more.
Grow your revenue with all-in-one prospecting solutions powered by the leader in private-company data.
But the “omics” — which include the fields of genomics, transcriptomics, metabolomics and proteomics, to name a few — could be the answer. The omics are a set of sciences that will allow us to code and dissect every cell, disease and tumor in the human body. The industry garnered $2.4 billion in funding in 2022, per Crunchbase data — on par with what it collected during the funding boom in 2021.
It can be tiring to hear someone wax poetic about the future of health care when progress is so slow-moving (“Why haven’t we cured cancer yet?” a friend asked me recently), but the ability to code all life will help us make drugs faster and help doctors treat patients more accurately.
Kairos Ventures, an early-stage biotech VC firm that brings startups out of universities, incubates several drug companies in their infancy that work to create more precise, efficiently produced drugs, such as Acutate Therapeutics and Linnaeus Therapeutics.
Alex Andrianopoulos, the chief research and development officer at the early-stage biotech VC firm, talked to Crunchbase News about the wide-reaching implications of budding biotech startups working in omics.
The following interview has been edited for length and clarity.
A lot of things somehow just happened all at once to really spur this industry. Genomics became very popular very fast. When did metabolomics, proteomics and transcriptomics start seeing more favor in the private markets?
Andrianopoulos: What we see in the private markets is translating all these discoveries from the ’70s, ’80s and ’90s into real technologies that could make a difference in pharmaceutical development as well as diagnostics in general.

It started with sequencers becoming affordable for researchers and startups. You didn’t need to have a whole floor of servers to actually transcribe the genome of a patient. If we can learn that much about human physiology from the genome, what else can we learn from ancillary structures and concepts?
This is critical. Since history started, we have been looking at health on a very experimental, very anecdotal basis. It was not based on understanding the programming of life. Now we know that if this gene is silenced, this disease will occur, or this cell will not grow properly.
We don’t understand why the human body produces cancers, and now we are starting to understand how we can treat cancer, but also why, in the first place, we have cancer.
I find when research goes from university to private markets, that signifies solid proof of concept. With the omics there is always a lot of promise, but we hear that with every advancement in science, and it feels like nothing has changed. When it comes to the end user, a patient, will they actually see the promised results of all this omics research in their lifetime?
Andrianopoulos: We don’t understand how the world used to be in the not-far past. You and I probably don’t remember how bad it was for cancer patients 20 or 30 years ago. The reason the average age of humans goes up on a constant basis is because of those improvements that are made on a daily basis by companies like those we invest in.
The study of the genome and the study of the proteome translate directly to better drugs that you know will work for a patient. It’s not a science-fiction picture I’m painting. I’m not talking about 50, 100 years into the future. I’m talking about a few decades.
Cancer, we have been calling one disease. But in reality it’s about 180 different diseases. So to understand the nature of the tumor, you can couple the drug to the patient’s genome and tumor to make it a much more effective treatment for that specific patient. That is what we mean with personalized medicine.
I am curious to know, going into 2023, if there are certain sectors in this area that you think are going to do better in the private markets or are going to do worse.
Andrianopoulos: The combination of AI with robotics will revolutionize the way we develop pharmaceuticals. And we’ll be producing better drugs combined with the data that we get from the omics domains, from the analysis of the genome, the proteome, the metabolome and so on.
Pharmaceuticals right now are very much done on a trial-and-error basis, with researchers saying “I believe this is the right path.” With AI and robotics we will be producing drugs at a much faster pace and on a much more targeted basis. Anything that involves AI in prediction of efficacy is going to be a big deal in the next few years in our space.
At the end of the day, all of this is up against a health care system, from which it’s such a pain to get a diagnosis for anything to begin with. Is there any worry about having something with so much potential being sullied once it goes through this filter of what is essentially a very difficult-to-resolve health care problem?
Andrianopoulos: You’re absolutely correct that people don’t want to touch our health care system. When a doctor prescribes a drug with the hope that it will work, sometimes it doesn’t work and it produces side effects and you have to go back to the hospital.
Most of the poor experiences we have today in the health care system are driven by the fact that our physicians are mostly guessing about what is wrong with us, and that produces a lot of failure. It’s the same thing with drug and drug development. The reason we have such a dramatic failure in the pipeline of drugs is because we guess at the very early stages.
What omics systems do is fill the gaps. The future is not going to be a doctor trying to figure out what this pain in the abdominal area means. Rather than having to guess, with a blood test, you can do genomic analysis to understand what the genome is doing on a single cell basis.
I have to tell you, (this is) a great era to live in. And a great era to be a VC in life sciences.
Illustration: Dom Guzman

Stay up to date with recent funding rounds, acquisitions, and more with the Crunchbase Daily.






![Illustration of a guy watering plants with a blocked hose - Global [Dom Guzman]](https://news.crunchbase.com/wp-content/uploads/quarterly-global-3-300x168.jpg)
67.1K Followers