I’ve spent a lot of time writing about the intricacies of the drug development process and intellectual property advancements being made to turn scientific discoveries into tangible tools humans can utilize to live a longer life.
But the process isn’t something that most people know much about, which makes it hard for the average reader to understand, critique and analyze advancements in the startup space.
Search less. Close more.
Grow your revenue with all-in-one prospecting solutions powered by the leader in private-company data.
Drug development is a slow, capital-intensive process. It takes around 10 years and a billion dollars to get a single drug to market, while 90% fail in the process of trying to reach that goal.
It would be far less risky to invest in almost any other kind of technology. However, over $70 billion has poured into the space so far this year alone (per Crunchbase data).
This is all in the pursuit of creating drugs that, in some cases, are far more profitable than other tech firms listed on the S&P 500.
But it can be hard to understand or critique the technological advancements we’re seeing in drug development if you don’t know how the process works to begin with. So let’s talk about what’s driving billions of dollars in the most risky startup sector in the world.
Drug discovery
The drug discovery process often begins in a university lab, where scientists are nose-deep in petri dishes looking for new strategies to get a drug into the body and test some of the infinite molecular compounds to see which ones could create a breakthrough drug.
Most of this research is powered via the National Institute of Health, a taxpayer-funded organization that contributes to almost every single drug that comes to market.
After a discovery, the university may choose to spin out a startup helmed by the leading researchers who are thrust, often for the first time, into the world of private enterprise where they look to raise their first round of funding.
Interest in drug discovery has increased since 2017, according to Crunchbase data. Around $1.6 billion has poured into the startup space so far this year, especially as the pharmaceutical industry tries to look for new targets—new parts of the cell for drugs to stick to.
Artificial intelligence is driving a lot of that startup growth. Leveraging a class of data collection technology that emerged with genomics becoming widespread, AI has the ability to look for new targets and simulate clinical trials, potentially reducing risk in drug investment.
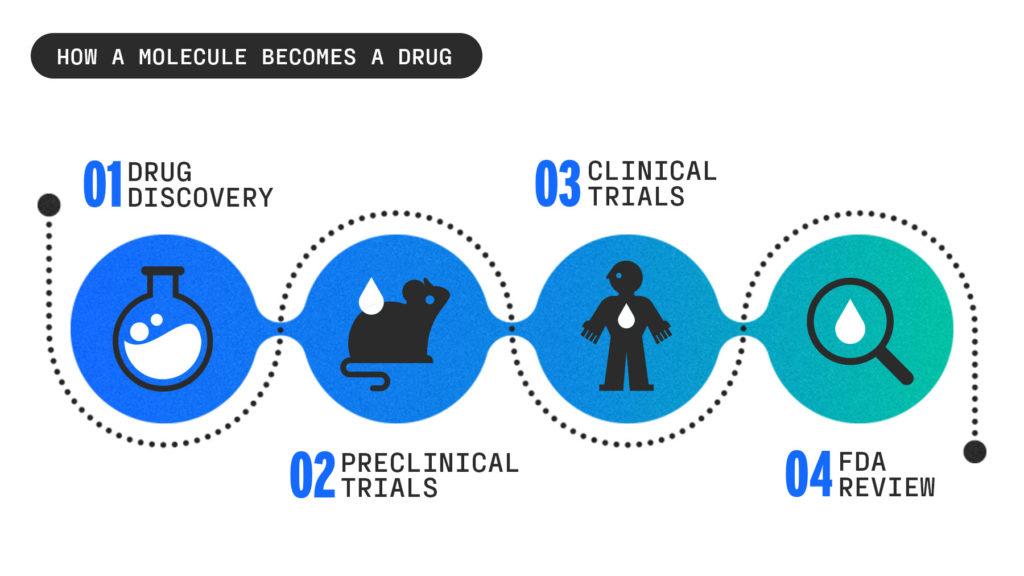
Preclinical trials
Once a startup identifies a handful of compounds that have the best chance of being effective, it moves into testing these compounds in labs and animals.
During this time, startups are doing IND-enabling studies, which is a formal request to the Food and Drug Administration to start dosing humans. This might also be the time a startup applies for an Orphan Drug or Breakthrough Therapy designation which are given to therapeutics aiming to address a large unmet need in the medical market to fast-track the process.
Preclinical trials are a necessary step to set dosing requirements and make sure drugs are safe before they enter humans. But they’re also extremely time-intensive for data that isn’t always accurate—petri dishes and animals aren’t the same as people.
Enter the organ-on-a-chip, a budding class of startups that construct a downsized version of a human liver, mimicking the environment of that organ. One of the largest studies on organ-chips found that they are able to better accurately reflect toxicity, a feat that could prevent death and permanent complications in human clinical trials.
While still in its early stages, organ-chip startups like Systemic Bio and CN Bio could cut costs and give drugs a better chance of making it through clinical trials. Since 2009, startups in this space have raised around $75 million, according to Crunchbase
This is also around the time startups begin raising early series funding, which is almost exclusively predicated on companies having strong research.
Clinical trials
Also called the Valley of Death, clinical trials are where most drugs meet their fate.
The time-consuming and expensive process got a much-needed makeover during the pandemic when COVID-19 shuttered clinical trial sites. Participating requires people to travel to clinical trial sites, often during work hours, for years at a time. Patients will drop out part way through studies which breeds diversity issues when studying drugs. If we don’t know how a drug may affect people with different lifestyles, dietary habits and sleep schedules, we can’t truly understand its safety.
For the first time, pharma companies could conduct clinical trials remotely using a slew of new technology that exploded onto the scene in 2021. Between 2020 and 2021, funding in the space went from $968 million to over $2 billion, according to Crunchbase data, as it became clear remote clinical trials were here to stay. They were by and large more effective, but it will take years before we understand the full scope of the impact.
Other startups, like Topography Health, help to find the right patients to join clinical trials. If your drug is meant to treat a specific kind of cancer at a specific stage of the cancer and requires qualifications, it’s difficult to comb through tens of thousands of patients to make sure they’re the right fit for a human study.
FDA review
The FDA approves most drugs based on three criteria: if it’s safe, if it’s effective and if it’s better than something that already exists. Sometimes the FDA will ask for additional studies to be conducted, and will continue monitoring the drug when it’s on the market.
Many of us take tiny little pills every day without knowing the intense risk that comes with taking them. Advancements in drug development may not sound as cool as consumer-focused technology, but they’re necessary to create drugs that are actually safe, and to do so as quickly as possible.
Over time, we’ve found the answers to diseases that were previously considered incurable. What’s next?
Illustration: Dom Guzman

Stay up to date with recent funding rounds, acquisitions, and more with the Crunchbase Daily.



![Illustration of 50+ woman on smartphone. [Dom Guzman]](https://news.crunchbase.com/wp-content/uploads/2021/01/Femtech_-300x168.jpg)
![Illustration of pandemic pet pampering. [Dom Guzman]](https://news.crunchbase.com/wp-content/uploads/2021/03/Pets-2-300x168.jpg)


67.1K Followers